Tiny robots created out of DNA may be the next big thing in cancer treatment.
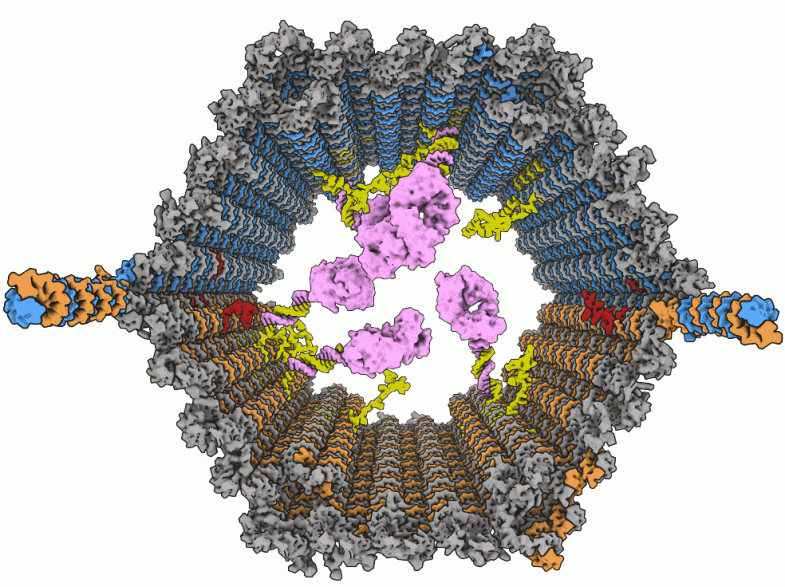
Wyss Institute scientists have developed a drug-delivering nanorobot that looks like an open-ended barrel (above). The exterior surface of the device is programmed to recognize a target on a cell surface; the drug payload (purple) is secured with anchor strands (yellow) to the interior. Double-stranded DNA latches (blue, red, and orange) ensure that the robot unlocks only in the presence of a molecular key expressed by the target cells. That opens the device (below right), enabling the payload to attack only the designated cells.
Image courtesy of Shawn M. Douglas / Wyss Institute for Biologically Inspired Engineering
In the not-so-distant future, a new kind of robot, one of the tiniest ever made, may have the ability to track down and destroy cancer cells.
Films like Fantastic Voyage (1966) and Innerspace (1987) have long conjured fictional images of microscopic submarines or machinery that can travel inside the human body to cure ailments. Now Shawn Douglas, a research fellow at Harvard’s Wyss Institute for Biologically Inspired Engineering, is working on making that a reality. In a recent issue of the journal Science, Douglas described a method for creating tiny machines—roughly the size of a virus—out of strands of protein and DNA.
As it turns out, says Douglas, DNA is an ideal material for building at the nanoscale level. Well-developed tools are already in place to understand, manipulate, and even manufacture it. Using computers and special machines called DNA synthesizers, it’s possible to create custom lengths of the molecule out of its four basic building blocks: adenine, cytosine, thymine, and guanine, chemicals known as nucleotides.“Our goal is to make tools that can zero in on malfunctioning cells,” he says. “We want to be able to fix things when they break—when cells go haywire due to cancer or other diseases where things just aren’t working correctly. To do that, I think it makes sense to master this kind of nanoscale construction.”
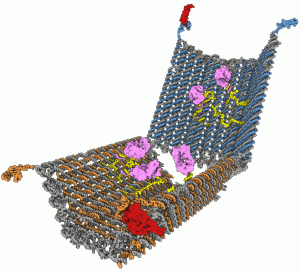
Wyss Institute scientists have developed a drug-delivering nanorobot that looks like an open-ended barrel (below left). The exterior surface of the device is programmed to recognize a target on a cell surface; the drug payload (purple) is secured with anchor strands (yellow) to the interior. Double-stranded DNA latches (blue, red, and orange) ensure that the robot unlocks only in the presence of a molecular key expressed by the target cells. That opens the device (above), enabling the payload to attack only the designated cells.
Image courtesy of Shawn M. Douglas / Wyss Institute for Biologically Inspired Engineering
To construct his devices, Douglas calls on these tools and a technique informally known as “DNA origami,” first developed by Caltech researcher Paul Rothemund in 2006. The process begins with a single long strand of DNA that Douglas uses as a backbone or “scaffold” for a structure. That strand is mixed with short chunks of custom-built DNA he calls “staples,” which are designed to bind to specific sections of the scaffold, bending and twisting it into pre-determined shapes.
Douglas chose to use the DNA of a virus called M13 (which is harmless to humans) as his scaffold, but notes that almost any long DNA molecule will work. “As long as you know the sequence [of those chemical building blocks],” he says, “it’s pretty simple to design molecular ‘staples’ that will pinch it together at specific spots.”
Each of Douglas’s nanorobots measures only 45 nanometers long by 35 nanometers wide—minuscule compared to the 75,000-nanometer width of an average human hair. The advantage of a machine this small, he says, is that it can directly interact with the surface of individual cells.
The nanorobots can also track down specific cell types, thanks to their molecular “latches.” Those two strands of twisted DNA fragments, known as aptimers, can be designed to react to a specific chemical marker, or ligand, on the surface of a particular cell type, such as those in a cancerous tumor. When they come in contact with that ligand, they bind to it and unfurl, allowing the device to open and release its contents. “It’s kind of like the space shuttle—you can put any payload you want in it,” Douglas explains. “What you use is going to depend on the application.”
At the moment, he is designing DNA aptimers that unravel in the presence of platelet-derived growth factor (PDGF), a protein that regulates cell growth and division. In cancerous cells, PDGF is over-expressed on the outer cell membrane, providing what is essentially a chemical beacon for his nanorobots.
Douglas hopes that by targeting specific cells in this way, his devices can help treat diseases like cancer without the need for traditional chemotherapy, which releases toxic agents throughout the body. “Those drugs might shut down cancer cells,” he says, “but they also shut down all sorts of good processes that we want to keep going,” leading to hair loss, nausea, and other side effects. His nanorobots, by contrast, could deliver medication directly to cancerous tumors, cutting down on the doses needed to reprogram or destroy rogue cells by delivering only tiny amounts directly to those cells themselves.
He is currently attempting to use nanorobots to attach antibodies to the surface of cancer cells, a process that could give the body’s immune system a way to identify and destroy those cells on its own. Initial experiments done in vitro look promising, and he hopes to accelerate production of the devices in order to use them for studies with lab animals within the next few years.
This video, presented courtesy of the Wyss Institute, explains how nanorobots work.
DNA nanorobot from Wyss Institute on Vimeo.